The most exciting and important electronic components are made from crystals called semiconductors. Depending on certain conditions, a semiconductor can act like a conductor or an insulator.
Silicon
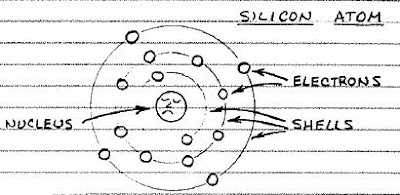
There are many different semiconducting materials, but silicon, the main ingredient of sand, is the most popular. A silicon atom has four electrons in its outermost shell, but it would like to have eight. Therefore, a silicon atom will link up with four of its neighbors to share electrons.
A cluster of silicon atoms sharing outer electrons forms a regular arrangement called a crystal. In above figure is a magnified view of a silicon crystal. To keep things simple, only the outer electrons of each atom are shown.
Silicon forms 27.7% of the earth's crust! only oxygen is more common. It's never found in the pure state. When purified, it's dark grey in color. Silicon and diamond share the same crystal structure and other properties. But silicon is not transparent. Silicon can be grown into big crystals. It's cut into wafers for making electronics parts.
Silicon Recipes
Pure silicon isn't very useful. That's why silicon makers spice up their silicon recipes with a dash of Phosphorus, Boron or other goodies. This is called doping the silicon. When grown into crystals, doped silicon has very useful electronic properties!
P & N Spiced Silicon Loaf
Boron, Phosphorus and certain other atoms can join with silicon atoms to form crystals. Here's the catch: A Boron atom has only three electrons in its outer shell. And a Phosphorus atom has five electrons in its outer shell. Silicon with extra Phosphorus electrons is called N-type silicon (n = negative). Silicon with electrons deficient Boron atoms is called P-type silicon (p = positive).
P-type silicon
A Boron atom in a cluster of silicon atoms leaves a vacant electron opening called a hole. It's possible for an electron from a nearby atom to "fall" into the hole. Therefore, the hole has moved to a new location. Remember, holes can move through silicon (just a bubbles move through water).
N-type silicon
A Phosphorus atom in a cluster of silicon atoms donates an extra electron. This extra electron can move through the crystal with comparative ease. In other words, n-type silicon can carry an electrical current. But so can p-type silicon! Holes "carry" the current.
Several example of electronic components that made from semiconductor is diode and transistor.
Several example of electronic components that made from semiconductor is diode and transistor.




The cute pics make me want to read this long explanation of semiconductor ^^
ReplyDeletethanks to share :D